Opposing effects of attention and consciousness on afterimages
Edited* by Anne Treisman, Princeton University, Princeton, NJ, and approved March 31, 2010 (received for review November 17, 2009)
Abstract
The brain's ability to handle sensory information is influenced by both selective attention and consciousness. There is no consensus on the exact relationship between these two processes and whether they are distinct. So far, no experiment has simultaneously manipulated both. We carried out a full factorial 2 × 2 study of the simultaneous influences of attention and consciousness (as assayed by visibility) on perception, correcting for possible concurrent changes in attention and consciousness. We investigated the duration of afterimages for all four combinations of high versus low attention and visible versus invisible. We show that selective attention and visual consciousness have opposite effects: paying attention to the grating decreases the duration of its afterimage, whereas consciously seeing the grating increases the afterimage duration. These findings provide clear evidence for distinctive influences of selective attention and consciousness on visual perception.
Sign up for PNAS alerts.
Get alerts for new articles, or get an alert when an article is cited.
Since the latter part of the past century, interest in the influences of selective attention and consciousness on perception has steadily increased. This discussion has raised the question of the relationship between attention and consciousness. By attention, we refer to selective perceptual attention and not vigilance or arousal; by consciousness, we refer to the content of consciousness (sometimes also referred to as awareness), and not to states of consciousness (e.g., wakefulness, dreamless sleep, or coma). Though some claim that both processes are inextricably connected (1–4), others suggest a certain level of independence (5–14). Psychophysical studies show that observers can pay attention to an invisible stimulus (15, 16), and that a stimulus can be clearly seen in the (near) absence of attention (4, 17). Though these data could be explained by arguing that these two processes covary and therefore any increase (respectively decrease) in one is associated with a similar but smaller increase (respectively decrease) in the other, this argument fails if attention and consciousness were to have opposing perceptual effects on the same stimulus. Finding such opponency would considerably strengthen the hypothesis that these processes are distinct (5).
Afterimage duration is a well-suited measure for the study of attention and consciousness. Changes in afterimage durations reflect the attentional and visibility manipulations during the afterimage induction phase. This permits the temporal separation of the attentional/visibility manipulations on the afterimage inducer and their subjective monitoring, and the measurement of the resultant effects (e.g., on afterimage appearance). This procedure effectively obviates the need for a simultaneous dual-task procedure.
Many afterimage and aftereffect studies are devoted to the influences of attention or consciousness in isolation. For example, removing stimuli from conscious content via various masking techniques that manipulate visibility decreases the motion aftereffect durations (18–20), tilt aftereffect (e.g., ref. 16, but see ref. 21), and face adaptation (22), and they do so as well for afterimages (23, 24; but see refs. 25–27). Similarly, attentional withdrawal decreases the size of the aftereffect for real (16, 28–30) and illusory lines (31), and for motion (32–36) and face (22) aftereffects. Curiously, though, attentional withdrawal seems to increase the duration of afterimages induced by real (37–40) and illusory adaptors (41). These latter findings beg the question: Could attention and consciousness affect perception in different and experimentally separable ways, even though up to now they have been found to work synergistically (4, 42)? Note that these previous findings cannot answer that question, because they have not been controlled for confounding concurrent changes in attention and consciousness levels. Therefore their interpretation in the present context is merely suggestive.
To our knowledge, no experiment to date has probed the simultaneous effects of both attention and consciousness on perception, while also controlling for possibly confounding stimulus and task changes (recently, one study [40] has gone a long way toward reducing confounding stimulus changes). Therefore, it is not known whether these two processes can induce different perceptual effects within a single controlled stimulus set, when all stimulus parameters and the task structure are identical, and when attention and visibility levels are not confounded.
We carefully investigated this issue, focusing on the formation of afterimages. We used methods that independently vary the amount of selective attention and stimulus consciousness. Therefore, even though until now, attention and consciousness have been shown to act synergistically (e.g., refs. 4 and 42), we are able to show that selective attention and stimulus consciousness can have different, even opposing, effects.
Results
Attention and Visibility Differently Affect Afterimage Duration.
In experiment 1, while manipulating attention via a demanding central task, we simultaneously manipulated the visibility of the stimulus via perceptual suppression. The independent manipulation of both allowed us to study high-attention and visible, low-attention and visible, high-attention and invisible, and low-attention and invisible conditions (Fig. 1A) using an identical adaptor stimulus and a single experimental paradigm.
Fig. 1.

Attention was manipulated by having subjects perform an attention-demanding central rapid serial visual presentation (RSVP) task (37, 43, 44; Materials and Methods) that drew attention away from the inducing stimulus, a gray Gabor patch (a Gaussian-windowed grating; i.e., inducer not/slightly attended), or having subjects report on the possible perceptual disappearances of the physically present Gabor (i.e., inducer highly attended). Note that throughout the text, we will refer to conditions where subjects attend to a central task as low-attention condition, in the sense that the amount of attention available for the adaptor is low (43). To maximize the difference between high-attention and low-attention conditions, subjects did not report the visibility of the Gabor when they were performing the RSVP task (i.e., no dual task) (31, 45). We independently manipulated the visibility of the afterimage inducer, which was always presented, by showing (or not) a strongly competing stimulus in the contralateral eye (23, 24). This continuous flash suppression (CFS) technique renders the Gabor perceptually invisible, even though the stimulus is physically present at the retina (Fig. 1B).
We first verified that our attentional manipulation worked. Average performance on the RSVP task was 54 ± 5% correct when the inducer was visible, and 47 ± 4% when the inducer was invisible (not significant, P > 0.15, two-tailed paired t test). Both measures were significantly higher than chance, 25% (both P < 0.0005, two-tailed t test), but also below 100%, indicating that the task was demanding. Only correct trials were included in the following analyses.
We found that afterimage duration (as indicated by the subjects’ button presses) depended on both attention and visibility: paying attention to the stimulus reduces afterimage duration (from mean ± SEM: 3.36 ± 0.29 s to 3.06 ± 0.35 s in visible conditions, and from 2.02 ± 0.43 s to 1.71 ± 0.42 s in invisible conditions), whereas visibility of the stimulus increases afterimage duration (from 1.71 ± 0.42 s to 3.06 ± 0.35 s in high-attention conditions, and from 2.02 ± 0.43 s to 3.36 ± 0.29 s in low-attention conditions; Fig. 1C). This observation is confirmed with a two-way repeated-measures ANOVA, which showed significant main effects of attention (P < 0.001) and visibility (P = 0.006), with no interaction (P > 0.9). Both of these effects were also significant in two-way ANOVAs in four individual subjects; an additional seven subjects showed a significant effect of visibility. Further support for separable influence of attention and consciousness comes from comparing the different conditions separately (Fig. 1D). We found that the attentional effects are significant in both visible and invisible conditions (both show a decrease of 300 ms, P < 0.021 and P < 0.014, respectively, paired one-tailed t test). Our observation that attention affects the processing of invisible stimuli is consistent with a recent fMRI study (46). Likewise, visibility effects are significant in both high- and low-attention conditions (increases of 1.4 s and 1.3 s, respectively, both P < 0.001, paired one-tailed t test). Furthermore, there is a strong correlation between afterimage duration in high- and low-attention conditions (Spearman rank correlation [over subjects]: ρ = 0.95; P < 0.001), and also between visible and invisible conditions (ρ = 0.75; P < 0.005), suggesting that the same underlying processes are responsible for the afterimage production in high- versus low-attention conditions, and visible versus invisible conditions.
Based on our task design, we believe eye movements were unlikely to influence these effects in experiment 1 (SI Materials and Methods; Fig. S1). We also confirmed that the results did not change when we excluded trials in which subjects reported not seeing any afterimage (SI Materials and Methods; Fig. S2). These data clearly show that attention and visibility can have opposite effects on visual perception, and that these effects do not interact significantly.
Control for Task Differences.
Although our comparison of high- and low-attention conditions was based on similar conditions, the attention task during the adaptation, as well as during the afterimage monitoring, differed (the subject had to remember a number in the low-attention condition, which was not required in the high-attention condition).
In experiment 2a, attention to the inducer was manipulated by making a single central RSVP task more or less difficult (47) (Materials and Methods). We confirmed that the attentional manipulation worked: performance on the RSVP task was 80 ± 8% in the easy task and 61 ± 5% in the hard task (P < 0.01, paired t test). Again, we found a significant effect of attention and visibility (Fig. 2). A two-way repeated-measures ANOVA showed significant effects of attention (P < 0.01) and visibility (P < 0.03), and no interaction (P > 0.15). The effect of attention was significant in both visible and invisible cases (P < 0.030 and P < 0.021, respectively, paired one-tailed t tests), and the effect of visibility was significant under both high and low attention (both P < 0.001). Therefore, when the task structure was kept identical, and only perceptual load on the central task differed, increased attention to the inducer reduced afterimage duration.
Fig. 2.

In experiment 2b, we reran experiment 2a on 10 subjects (of which four participated in the previous version as well) while monitoring their eye movements. Eye movements can potentially influence our data in three ways: (i) small eye-movements will jitter the stimulus on the retina, thereby decreasing adaptation (48) and consequently reducing afterimage duration; (ii) subjects might keep fixation at the central task when it is difficult, while fixating the peripheral stimulus when the central task is easy, potentially introducing a confound; and (iii) eye movements during the induction phase might cause the interocular suppression to fail (49), thereby making the stimulus visible when it should be invisible. When analyzing subjects’ eye movements, we found no correlation between SDs in eye position parallel or orthogonal to the stimulus orientation and the afterimage duration, (control for point i above; SI Materials and Methods). We excluded trials in which subjects did not fixate the fixation mark, and trials in which a saccade was detected (control for points ii and iii). With these controls in place, the results (Fig. S3) confirm that attention decreased afterimage duration, whereas visibility increased afterimage duration [repeated-measures ANOVA: main effects of attention (P = 0.03) and visibility (P = 0.005), with no interaction (P > 0.25)].
Attention and Visibility Effects over a Range of Contrasts.
Are our visibility findings simply due to the strong interocular suppressor? In experiment 3, we measured the effects of attention and visibility on afterimage duration while varying the contrast of the suppressing CFS. The CFS contrast ranged from 0% (i.e., no CFS) to perithreshold (1.6–6.3%) to suprathreshold (12.5–100%) contrast values; the inducing Gabor patch contrast was fixed at 34%.
The effects of CFS (compared with the 0% contrast, no CFS condition) are significant (P < 0.05, one-tailed t test) for contrasts >6% and >12% for high- and low-attention conditions, respectively (Fig. 3A), which is also the contrast when it was strong enough to cause visibility changes in the inducer stimulus. The effect of increased attention (ΔAI) was significant (i.e., P < 0.05, one-tailed t test) for CFS with 0% contrast, and for contrasts >12% (Fig. 3B). Therefore, our conclusions in experiment 1 are not just the result of the specific settings of the CFS stimulus.
Fig. 3.

We fail to see significant attentional effects with CFS at perithreshold contrasts of 1.6–6.3% (Fig. 3B). It is now well established that stimuli around the detection threshold attract attention (50, 51). Therefore, the perithreshold CFS stimuli probably attracted the subjects’ attention, even when the distracting RSVP task was performed. This explains why at these contrasts the low-attention conditions fall on top of the attended conditions (as if they were “highly attended” conditions; Fig. 3A).
Controls for Stimulus Differences.
Could the mere presence or absence of the CFS stimulus have caused our visibility effects? Conceivably, interocular masking could increase contrast adaptation, thereby increasing detection thresholds and, ultimately, reducing afterimage durations (40, 52, 53). However, we already showed that qualitatively identical and significant results are obtained at CFS mask Michelson contrasts as low as ∼10% (see previous section). To more stringently control for the possible influences of CFS and contrast threshold increases on visibility, we performed an experiment in which subjects always viewed the same Gabor, and the same CFS mask.
In experiment 4, the contrast of the CFS mask was determined for each subject, such that in about half of all trials the afterimage-inducing Gabor patch was perceptually invisible, whereas in the remaining half the Gabor was visible to a variable extent. Because all conditions were otherwise identical (including the CFS stimulus), the sole variant was perceptual visibility. The data confirmed the previous experiments: visible trials led to longer afterimages (1.79 ± 0.23 s) than invisible trials (1.52 ± 0.27 s, P = 0.02, paired one-tailed t test, also significant in 8/10 individual subjects; Fig. 4A). An auxiliary linear regression (Fig. 4B; SI Materials and Methods) showed a slope of 0.13 (R2 = 0.96, P < 0.001), leading to a 0.51-s increase in afterimage duration for 4 s—the trial duration—of visibility. Furthermore, there was a significant correlation between the individual subjects’ effects of visibility in this experiment and in experiment 1 (ρ = 0.67, P < 0.05), suggesting that the same process was at work.
Fig. 4.
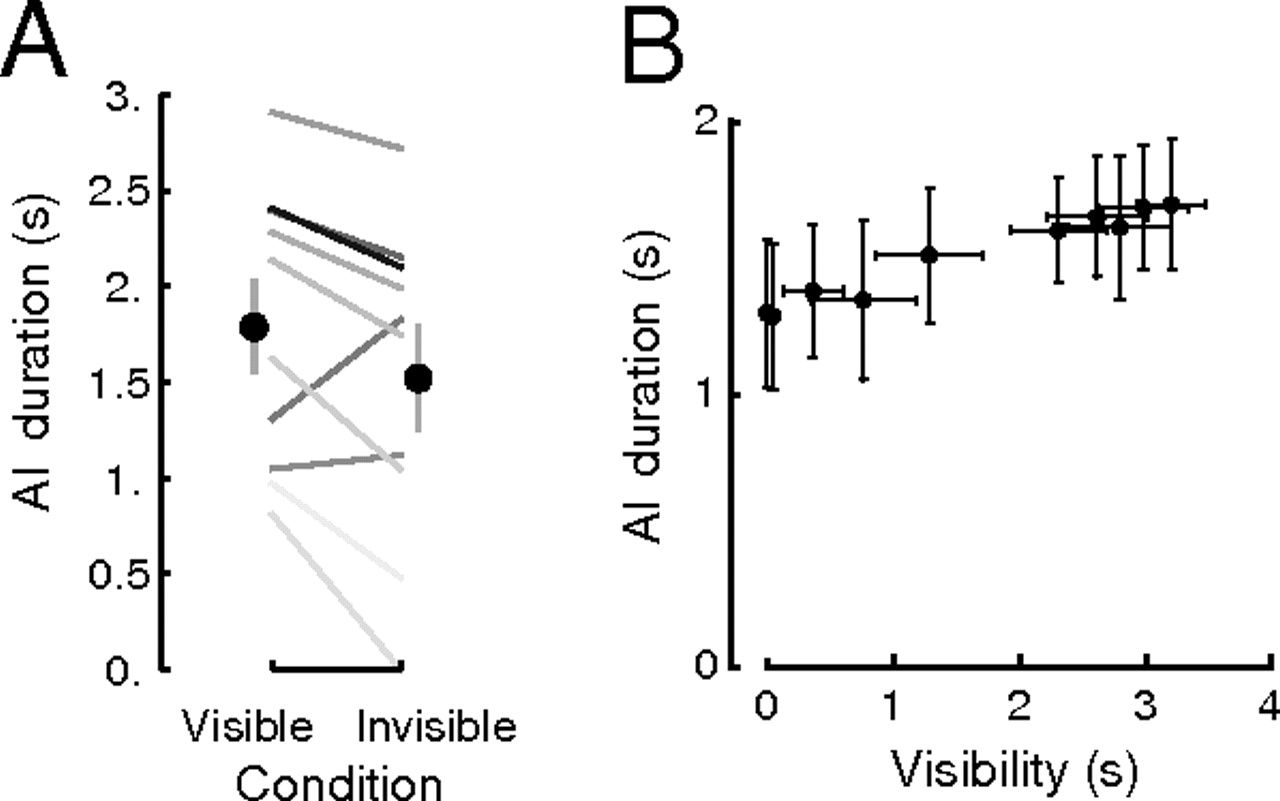
In experiment 5, we removed the CFS stimulus altogether, and decreased the inducer contrast to 6%, such that intermittent peripheral Troxler fading occurred (54). This fading caused trial-by-trial variations in stimulus visibility. Again we found that the longer the perceptual visibility of the inducer, the longer the afterimage (Fig. 5, linear regression: R2 = 0.8, P < 0.005, slope = 0.25, leading to a 1.0-s increase in afterimage duration for 4 s—the trial duration—of visibility).
Fig. 5.

Experiments 4 and 5 confirm that perceptual visibility (i.e., whether the subject is conscious of the stimulus) is a significant factor in afterimage formation above and beyond the physical presence of the adaptor. The presence or absence of CFS affects afterimage duration mainly, although perhaps not solely, through the manipulation of perceptual visibility.
Discussion
The relationship between selective attention and consciousness has been much debated since the 19th century. We here address this important question via a direct comparison on the basis of a full-factorial 2 × 2 design of afterimage perception, while controlling for the effects of stimulus and task differences, and eye movements. We show that paying more attention to the inducer invariably shortens afterimage duration, while increasing the visibility of the inducer increases afterimage duration compared with invisibility. Clearly, selective attention and stimulus consciousness have separable effects on perception, as reviewed previously (5, 7), and, in the context of afterimages, may even have opposite effects. It will be important to investigate the extent to which such a full-factorial design can show dissociations between attention and consciousness for other perceptual phenomena [a recent study used a full-factorial design in priming (55), but did not observe opposite effects of attention and consciousness].
Why would selective attention and consciousness have opposing effects when so often both act synergistically (4, 42)? What seems paradoxical is that attending to the inducer will reduce the duration of the afterimage. The effects of consciousness (visibility) are straightforward to explain: visible inducers evoke larger neuronal activity during induction of afterimages than invisible ones, resulting in stronger and longer afterimages, compared with invisible inducers. This is consistent with other forms of aftereffects (16, 18–20, 22) and with general findings in neurophysiology (e.g., ref. 56).
The counterintuitive nature of the attentional effects is a bit more difficult to explain. Suzuki and Grabowecky (37) suggested that afterimages are the result of adaptation in two stages. The first stage is sensitive to the contrast polarity of the image. This stage cares about the spatial relationship between dark and light regions of a stimulus (it could, for example, increase its response when presented with a patch that is dark on one side and light on the other side, but not to a patch with the reverse contrast polarity). This stage is the source of the afterimage, because when it is adapted and stimulated with a neutral stimulus, it will produce an afterimage of opposite polarity to the afterimage inducer. In Suzuki and Grabowecky's framework, this stage is not critically affected by attention. Although a later study (40) did indicate that both attention and consciousness change adaptation states at this level, Suzuki and Grabowecky's general framework remains plausible.
The second stage is not sensitive to the contrast polarity of the image. It will be activated by a patch that is dark on one side and light on the other side, and also a patch of reverse contrast-polarity, as long as the stimulus has the preferred orientation and position. This stage will modulate the strength of the subsequently perceived afterimage (40, 52, 53): when activity in this stage is high, the afterimage is strong, when activity is low, the afterimage is weak or absent. During the adaptation phase, polarity-insensitive cells will adapt more in the presence of selective attention, reducing their activity during the afterimage phase. Subsequently, this will cause the afterimages to be weaker if attention is paid during the induction phase. In this scheme, adaptation works in opposite directions in polarity-sensitive and polarity-insensitive cells. The opposite effects of attention and consciousness in our experiments can be explained if they affect adaptation at these levels differently. A computational model based on such principles reproduces the psychophysical effects of attention (38). Neurophysiological studies also support this idea: though attention modulates the firing rates of neurons in early visual areas (57, 58), perceptual invisibility in binocular rivalry has little effect on their firing activities (59–63). Clearly, attention and consciousness have different ways of influencing early visual activity, which, as we have pointed out, may cause opposing effects on afterimage duration.
There is at least one other explanation for why attention shows these paradoxical effects. We assume a single stage that is more adapted when attention is directed to the stimulus. By itself, this predicts a stronger afterimage in high-attention condition. We furthermore assume that the afterimage decays with a time constant of several seconds (25, 64), and that increased levels of selective attention reduce this time constant. This means that a neuron that has been modulated by increased levels of attention will return quicker from an adapted state to baseline (i.e., reduced time constants) than a neuron not modulated by attention. The shunting effects of synaptic conductance changes (65), in combination with local excitatory feedback, gives rise to effective time constants in the range of seconds (66, 67), which has the correct order of magnitude to underlie afterimages and the seemingly paradoxical effect of attention.
Although detrimental effects of attention are rare, they are not unheard of. Other examples of negative influences of attention include the expression of overlearnt skills in motor learning (68, 69), texture segregation (70), and recognition memory (71). One commonality among all four is the involvement of peripheral neuronal structures that might well be amenable to modulation by selective attention but not by consciousness. In that sense, our findings are fully compatible with the predictions of global workspace theories of consciousness (7, 72).
When attention is withdrawn, even salient stimuli can become perceptually invisible. From that perspective it may seem puzzling that we observed opposite effects when comparing low attentional processing with invisibility due to perceptual rivalry. A failure to report on a stimulus that is physically present can be induced by different psychophysical techniques (73). In particular, invisibility can be induced by perceptual suppression, such as CFS (e.g., ref. 23) or backward masking (e.g., ref. 74), or by inattention, such as inattentional blindness (4) or change blindness (42). Though the neurophysiological operations underlying perceptual invisibility remain unclear, some evidence suggests that perceptual suppression and inattentional blindness are supported by similar mechanisms (75, 76). Our findings are inconsistent with such a framework, arguing instead for distinct mechanisms. Furthermore, a recent report (77) demonstrated that subjects’ confidently report stimulus absence (i.e., miss) during perceptual suppression but not during attentional distraction. The results were quantified using type-2 signal detection theory (78). These studies imply different neuronal mechanisms for perceptual suppression (possibly due to suppressed sensory activity in the ventral visual pathway) (76) and for inattentional invisibility (possibly due to a failure of top-down attentional amplification of sensory signals from frontal-parietal cortex) (7, 75).
Our experiments show that selective attention and stimulus consciousness affect the perception of afterimages differently, and even oppositely. As pointed out previously (74), this makes it all the more critical to distinguish the neuronal correlates of selective attention from those of the current content of consciousness.
Materials and Methods
Stimuli.
Adaptation was induced by presenting a 0.23 cycles/°, 34% Michelson contrast, Gaussian-windowed (σ = 1.43°), randomly oriented grating, located 4.9° peripherally. Presentation was monocular, and balanced over the eyes over trials. This Gabor was always presented throughout the induction period (4 s). The suppressor stimulus was a Gaussian-windowed (σ = 1.43°) checkerboard (0.78 cycles/°) which rotated at 150°/s, and reversed contrast every 67 ms. The contrast of this suppressor stimulus was 100% in experiments 1, 2a, and 2b, systematically varied in experiment 3, tailored for each subject in experiment 4 (for 8/10 of the subjects it was set to 9%, for 2/10 subjects it was set to 20%), and 0% for experiment 5. At subsequent trials, the Gabor was shifted around the fixation dot in counterclockwise fashion by 45°, preventing repeated adaptation at a single location. Depending on afterimage durations, and delays between subsequent trials, this leads to at least 30 s (generally >50 s) of deadaptation between adaptation periods at identical locations. Background luminance was 49 cd/m2. All experiments were performed on a gamma-corrected monitor.
Procedure.
In experiment 1, in the low-attention trials, attention was distracted from the afterimage-inducing stimulus by having subjects perform a rapid serial visual presentation (RSVP) task. We used RSVP of letters (red Helvetica 12-point), which were shown (133 ms, no interletter interval) within the boundaries of the fixation dot. Subjects had to count the number of Xs (n = 2–5), which was reported after the afterimage had disappeared. In the high-attention trials, the RSVP task was not performed (but the letters were shown). Instead, subjects tracked the subjective visibility of the inducer by pressing and releasing a button. Subjects did not report visibility in the low-attention trials, avoiding the need to employ attention to the inducer (in standard dual-task paradigms, subjects are forced to attend to the stimulus). After the adaptation period, subjects indicated the duration of the afterimage with a button press and release. In experiments 1, 2a, 3, 4, and 5, when subjects perceived no afterimage, they pressed a separate button, in which case the duration of the afterimage was recorded as being 0 s in duration. Experiment 2b had no such button, and subjects merely had to quickly release the button with which the afterimage duration was indicated.
In experiment 2a and 2b, we manipulated attentional load. The attention-demanding central RSVP task was to count the number of times (n = 1, 2, 3, or 4) a cross (height: 1.9°, width: 1.4°) of a particular orientation and color appeared. Crosses were presented at fixation for 133 ms and blanked for 133 ms. Two target crosses were at least separated by one other cross. The easy task was to count the upright and inverted red crosses. The hard task was to count the number of upright yellow and inverted green crosses (but not the opposite conjunction). According to the load theory of attention (43), in trials with the easy task the observer had more “free” attention to direct to the inducer than in trials with the hard task. Therefore, without changing the task or the stimuli, we could change the amount of attention paid to the afterimage inducer. An additional advantage of this experiment is that eye movements are highly unlikely to differ between the different conditions (47), which we empirically confirmed in experiment 2b.
Information on the number of trials, the trial inclusion criteria, and the eye movements can be found in SI Materials and Methods.
Acknowledgments
The authors thank April Kartikasari and Jan Brascamp for comments on the manuscript, and Jan Brascamp and Tomas Knapen for insightful discussions. Support for this research was provided by a Rubicon grant of the Netherlands Organisation of Scientific Research (NWO; to J.J.A.v.B.), the National Science Foundation, the Mathers Foundation, The Gimbel Fund, and the WCU program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (R31-2008-000-10008-0) (C.K.), and the Japan Society for the Promotion of Science (N.T.).
Supporting Information
Supporting Information (PDF)
Supporting Information
- Download
- 180.88 KB
References
1
JK O'Regan, A Noë, A sensorimotor account of vision and visual consciousness. Behav Brain Sci 24, 939–973 (2001).
2
MI Posner, Attention: The mechanisms of consciousness. Proc Natl Acad Sci USA 91, 7398–7403 (1994).
3
PM Merikle, S Joordens, Parallels between perception without attention and perception without awareness. Conscious Cogn 6, 219–236 (1997).
4
A Mack, I Rock Inattentional Blindness (MIT Press, Cambridge, MA, 1998).
5
C Koch, N Tsuchiya, Attention and consciousness: Two distinct brain processes. Trends Cogn Sci 11, 16–22 (2007).
6
W Wundt Grundzüge der Physiologischen Psychologie (Engelmann, Leipzig, 1874).
7
S Dehaene, JP Changeux, L Naccache, J Sackur, C Sergent, Conscious, preconscious, and subliminal processing: A testable taxonomy. Trends Cogn Sci 10, 204–211 (2006).
8
VA Lamme, Why visual attention and awareness are different. Trends Cogn Sci 7, 12–18 (2003).
9
GF Woodman, SJ Luck, Dissociations among attention, perception, and awareness during object-substitution masking. Psychol Sci 14, 605–611 (2003).
10
RW Kentridge, CA Heywood, L Weiskrantz, Spatial attention speeds discrimination without awareness in blindsight. Neuropsychologia 42, 831–835 (2004).
11
N Block, Two neural correlates of consciousness. Trends Cogn Sci 9, 46–52 (2005).
12
T Bachmann, A single metatheoretical framework for a number of conscious-vision phenomena. Psychological Science Around the World, ed Q Jing (Psychology Press, London), pp. 229–242 (2006).
13
A Schurger, A Cowey, JD Cohen, A Treisman, C Tallon-Baudry, Distinct and independent correlates of attention and awareness in a hemianopic patient. Neuropsychologia 46, 2189–2197 (2008).
14
A Treisman, Consciousness and perceptual binding. The Unity of Consciousness, ed A Cleeremans (Oxford Univ Press, London, 2003).
15
L Naccache, E Blandin, S Dehaene, Unconscious masked priming depends on temporal attention. Psychol Sci 13, 416–424 (2002).
16
R Kanai, N Tsuchiya, FA Verstraten, The scope and limits of top-down attention in unconscious visual processing. Curr Biol 16, 2332–2336 (2006).
17
L Fei-Fei, R VanRullen, C Koch, P Perona, Rapid natural scene categorization in the near absence of attention. Proc Natl Acad Sci USA 99, 9596–9601 (2002).
18
K Maruya, H Watanabe, M Watanabe, Adaptation to invisible motion results in low-level but not high-level aftereffects. J Vis 8, 1–11 (2008).
19
R Blake, D Tadin, KV Sobel, TA Raissian, SC Chong, Strength of early visual adaptation depends on visual awareness. Proc Natl Acad Sci USA 103, 4783–4788 (2006).
20
H Wiesenfelder, R Blake, The neural site of binocular rivalry relative to the analysis of motion in the human visual system. J Neurosci 10, 3880–3888 (1990).
21
NJ Wade, P Wenderoth, The influence of colour and contour rivalry on the magnitude of the tilt after-effect. Vision Res 18, 827–835 (1978).
22
F Moradi, C Koch, S Shimojo, Face adaptation depends on seeing the face. Neuron 45, 169–175 (2005).
23
N Tsuchiya, C Koch, Continuous flash suppression reduces negative afterimages. Nat Neurosci 8, 1096–1101 (2005).
24
LA Gilroy, R Blake, The interaction between binocular rivalry and negative afterimages. Curr Biol 15, 1740–1744 (2005).
25
C Hofstoetter, C Koch, DC Kiper, Motion-induced blindness does not affect the formation of negative afterimages. Conscious Cogn 13, 691–708 (2004).
26
LC Lack Selective Attention and the Control of Binocular Rivalry (Mouton, The Hague, 1978).
27
WJK Craik, Origin of afterimages. Nature 148, 512 (1940).
28
MJ Spivey, MJ Spirn, Selective visual attention modulates the direct tilt aftereffect. Percept Psychophys 62, 1525–1533 (2000).
29
T Liu, J Larsson, M Carrasco, Feature-based attention modulates orientation-selective responses in human visual cortex. Neuron 55, 313–323 (2007).
30
D Melcher, Selective attention and the active remapping of object features in trans-saccadic perception. Vision Res 49, 1249–1255 (2009).
31
L Montaser-Kouhsari, R Rajimehr, Attentional modulation of adaptation to illusory lines. J Vis 4, 434–444 (2004).
32
A Rezec, B Krekelberg, KR Dobkins, Attention enhances adaptability: Evidence from motion adaptation experiments. Vision Res 44, 3035–3044 (2004).
33
A Chaudhuri, Modulation of the motion aftereffect by selective attention. Nature 344, 60–62 (1990).
34
D Alais, R Blake, Neural strength of visual attention gauged by motion adaptation. Nat Neurosci 2, 1015–1018 (1999).
35
G Rees, CD Frith, N Lavie, Modulating irrelevant motion perception by varying attentional load in an unrelated task. Science 278, 1616–1619 (1997).
36
I Mukai, T Watanabe, Differential effect of attention to translation and expansion on motion aftereffects (MAE). Vision Res 41, 1107–1117 (2001).
37
S Suzuki, M Grabowecky, Attention during adaptation weakens negative afterimages. J Exp Psychol Hum Percept Perform 29, 793–807 (2003).
38
J Wede, G Francis, Attentional effects on afterimages: Theory and data. Vision Res 47, 2249–2258 (2007).
39
L Lou, Effects of voluntary attention on structured afterimages. Perception 30, 1439–1448 (2001).
40
JW Brascamp, JJ van Boxtel, T Knapen, R Blake, A dissociation of attention and awareness in phase-sensitive but not phase-insensitive visual channels. J Cogn Neurosci, 10.1162/jocn.2009.21397. (2010).
41
A Lak, Attention during adaptation weakens negative afterimages of perceptually colour-spread surfaces. Can J Exp Psychol 62, 101–109 (2008).
42
RA Rensink, JK O'Regan, JJ Clark, To see or not to see: The need for attention to perceive changes in scenes. Psychol Sci 8, 368–373 (1997).
43
N Lavie, Perceptual load as a necessary condition for selective attention. J Exp Psychol Hum Percept Perform 21, 451–468 (1995).
44
DK Lee, L Itti, C Koch, J Braun, Attention activates winner-take-all competition among visual filters. Nat Neurosci 2, 375–381 (1999).
45
A Pastukhov, J Braun, Perceptual reversals need no prompting by attention. J Vis 7, 1–17 (2007).
46
B Bahrami, N Lavie, G Rees, Attentional load modulates responses of human primary visual cortex to invisible stimuli. Curr Biol 17, 509–513 (2007).
47
B Bahrami, D Carmel, V Walsh, G Rees, N Lavie, Unconscious orientation processing depends on perceptual load. J Vis 8, 1–10 (2008).
48
S Martinez-Conde, SL Macknik, XG Troncoso, TA Dyar, Microsaccades counteract visual fading during fixation. Neuron 49, 297–305 (2006).
49
LC van Dam, R van Ee, Retinal image shifts, but not eye movements per se, cause alternations in awareness during binocular rivalry. J Vis 6, 1172–1179 (2006).
50
Y Tsushima, Y Sasaki, T Watanabe, Greater disruption due to failure of inhibitory control on an ambiguous distractor. Science 314, 1786–1788 (2006).
51
Y Tsushima, AR Seitz, T Watanabe, Task-irrelevant learning occurs only when the irrelevant feature is weak. Curr Biol 18, R516–R517 (2008).
52
LE Leguire, R Blake, Role of threshold in afterimage visibility. J Opt Soc Am 72, 1232–1237 (1982).
53
MA Georgeson, RS Turner, Afterimages of sinusoidal, square-wave and compound gratings. Vision Res 25, 1709–1720 (1985).
54
IPV Troxler, Über das Verschwinden gegebener Gegenstände innerhalb unseres Gesichtskreises. Ophthalmologische Bibliothek, eds K Himly, JA Schmidt (Springer, Jena, Germany) 2, 1–53 (1804).
55
E Van den Bussche, G Hughes, NV Humbeeck, B Reynvoet, The relation between consciousness and attention: An empirical study using the priming paradigm. Conscious Cogn 19, 86–97 (2010).
56
S Dehaene, et al., Cerebral mechanisms of word masking and unconscious repetition priming. Nat Neurosci 4, 752–758 (2001).
57
BC Motter, Focal attention produces spatially selective processing in visual cortical areas V1, V2, and V4 in the presence of competing stimuli. J Neurophysiol 70, 909–919 (1993).
58
CJ McAdams, RC Reid, Attention modulates the responses of simple cells in monkey primary visual cortex. J Neurosci 25, 11023–11033 (2005).
59
P Fries, PR Roelfsema, AK Engel, P Konig, W Singer, Synchronization of oscillatory responses in visual cortex correlates with perception in interocular rivalry. Proc Natl Acad Sci USA 94, 12699–12704 (1997).
60
DA Leopold, NK Logothetis, Activity changes in early visual cortex reflects monkeys' percepts during binocular rivalry. Nature 379, 549–553 (1996).
61
SR Lehky, JH Maunsell, No binocular rivalry in the LGN of alert macaque monkeys. Vision Res 36, 1225–1234 (1996).
62
A Maier, et al., Divergence of fMRI and neural signals in V1 during perceptual suppression in the awake monkey. Nat Neurosci 11, 1193–1200 (2008).
63
M Wilke, KM Mueller, DA Leopold, Neural activity in the visual thalamus reflects perceptual suppression. Proc Natl Acad Sci USA 106, 9465–9470 (2009).
64
DH Kelly, E Martinez-Uriegas, Measurements of chromatic and achromatic afterimages. J Opt Soc Am A 10, 29–37 (1993).
65
C Koch Biophysics of Computation (Oxford Univ Press, London, 1999).
66
RJ Douglas, C Koch, M Mahowald, KA Martin, HH Suarez, Recurrent excitation in neocortical circuits. Science 269, 981–985 (1995).
67
XJ Wang, Probabilistic decision making by slow reverberation in cortical circuits. Neuron 36, 955–968 (2002).
68
SL Beilock, TH Carr, C MacMahon, JL Starkes, When paying attention becomes counterproductive: Impact of divided versus skill-focused attention on novice and experienced performance of sensorimotor skills. J Exp Psychol Appl 8, 6–16 (2002).
69
GD Logan, MJC Crump, The left hand doesn't know what the right hand is doing: The disruptive effects of attention to the hands in skilled typewriting. Psychol Sci 20, 1296–1300 (2009).
70
Y Yeshurun, M Carrasco, Attention improves or impairs visual performance by enhancing spatial resolution. Nature 396, 72–75 (1998).
71
JL Voss, KA Paller, An electrophysiological signature of unconscious recognition memory. Nat Neurosci 12, 349–355 (2009).
72
BJ Baars, The conscious access hypothesis: Origins and recent evidence. Trends Cogn Sci 6, 47–52 (2002).
73
CY Kim, R Blake, Psychophysical magic: Rendering the visible 'invisible'. Trends Cogn Sci 9, 381–388 (2005).
74
PU Tse, S Martinez-Conde, AA Schlegel, SL Macknik, Visibility, visual awareness, and visual masking of simple unattended targets are confined to areas in the occipital cortex beyond human V1/V2. Proc Natl Acad Sci USA 102, 17178–17183 (2005).
75
G Rees, Neuroimaging of visual awareness in patients and normal subjects. Curr Opin Neurobiol 11, 150–156 (2001).
76
C Keysers, DI Perrett, Visual masking and RSVP reveal neural competition. Trends Cogn Sci 6, 120–125 (2002).
77
R Kanai, C-H Tseng, S-W Wang, V Walsh, A distinction between perceptual blindness and attentional blindness (I): Low-contrast versus attentional distraction. J Vis 9, 122, (abstr), 10.1167/9.8. (2009).
78
C Kunimoto, J Miller, H Pashler, Confidence and accuracy of near-threshold discrimination responses. Conscious Cogn 10, 294–340 (2001).
Information & Authors
Information
Published in
Classifications
Submission history
Published online: April 27, 2010
Published in issue: May 11, 2010
Keywords
Acknowledgments
The authors thank April Kartikasari and Jan Brascamp for comments on the manuscript, and Jan Brascamp and Tomas Knapen for insightful discussions. Support for this research was provided by a Rubicon grant of the Netherlands Organisation of Scientific Research (NWO; to J.J.A.v.B.), the National Science Foundation, the Mathers Foundation, The Gimbel Fund, and the WCU program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (R31-2008-000-10008-0) (C.K.), and the Japan Society for the Promotion of Science (N.T.).
Notes
*This Direct Submission article had a prearranged editor.
Authors
Competing Interests
The authors declare no conflict of interest.
Metrics & Citations
Metrics
Altmetrics
Citations
Cite this article
Opposing effects of attention and consciousness on afterimages, Proc. Natl. Acad. Sci. U.S.A.
107 (19) 8883-8888,
http://doi.org/10.1073/pnas.0913292107
(2010).
Copied!
Copying failed.
Export the article citation data by selecting a format from the list below and clicking Export.
Cited by
Loading...
View Options
View options
PDF format
Download this article as a PDF file
DOWNLOAD PDFLogin options
Check if you have access through your login credentials or your institution to get full access on this article.
Personal login Institutional LoginRecommend to a librarian
Recommend PNAS to a LibrarianPurchase options
Purchase this article to access the full text.
